Background: Carfilzomib, lenalidomide and dexamethasone (KRd) has been shown to be an effective induction regimen for newly diagnosed myeloma (NDMM), particularly in myeloma with high-risk (HR) features in the FORTE trial (Mina et al, Lancet Onc 2023). Here, we present a retrospective analysis in patients with HR NDMM in a real-world population from two high-volume centers, highlighting efficacy and survival outcomes in high-risk subgroups to gain a better understanding of the utility of this regimen in clinical practice.
Methods: We conducted a retrospective study of patients with HR NDMM treated with KRd at Memorial Sloan Kettering (MSK) and Winship Cancer Institute from 1/1/2015 to 9/30/22. Cutoff date for analysis was 12/31/2022. HR was defined as the presence of high-risk cytogenetic abnormalities (HRCA) including +1q, del(1p), t(4;14), t(14;16), t(14;20), and/or del(17p), circulating plasma cells (cPC) ≥5%, extramedullary disease (EMD), and/or complex cytogenetics by conventional cytogenetics. Patients who received ≤1 prior cycle of a different MM regimen were included. Responses and progression were evaluated per International Myeloma Working Group Uniform Response Criteria. Discrete patient characteristics were summarized by frequency (percentage) and continuous characteristics were summarized by median (Interquartile Range, IQR). Progression-free survival (PFS) and overall survival (OS) were evaluated by Kaplan-Meier method. Association between time to event outcomes and patient's characteristics were determined by log-rank test. Univariate Cox proportional hazard model was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: We identified 179 consecutive NDMM patients with HR features treated with KRd at MSK (N=106) and Winship Cancer Institute (N=73). Baseline patient characteristics are listed in Table 1. The median age was 62 (IQR, 55-68) and 28% of patients were Black. There were 45 (25%), 76 (42%), 47 (26%), and 11 (6%) patients with 0, 1, 2, 3+ HRCA, respectively. A median of 5 (IQR 4-6) cycles were administered. At data cutoff, 70% of patients received upfront autologous stem cell transplant (ASCT). Best overall response rate (ORR) by the end of KRd induction was 94% for 178 response-evaluable patients, including 39% with ≥complete response (CR) and 78% with ≥very good partial response (VGPR).
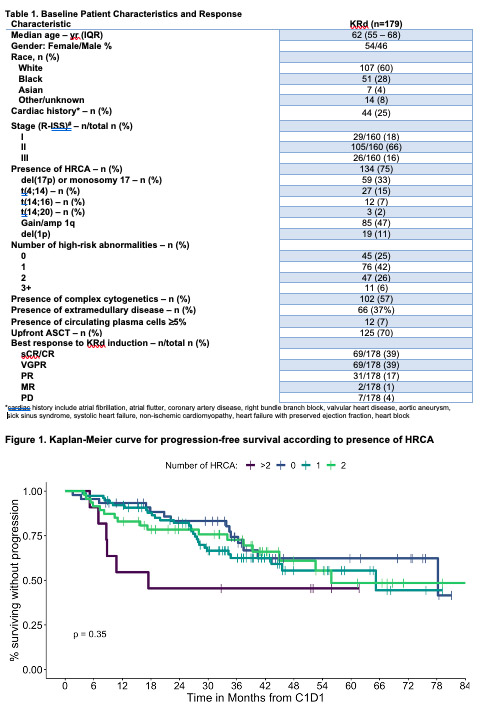
After a median follow-up of 41.9 (95% CI 39-45.6) months, the median PFS (mPFS) was 78.2 months (95% CI 52.5-not reached [NR]), and 2-year estimated PFS was 79% (73%-85%). Median OS (mOS) was not reached, and 2-year OS was 92% (95%CI 89%-97%). For HR subgroups based on the number of HRCA present, mPFS was 78.2 (95% CI 40.1-NR), 65.2 (43.4-NR), 55.9 (44.9-NR), and 17.5 (8.78-NR) months for patients with 0, 1, 2, 3+ HRCA, respectively, with 2-year PFS of 83% (95% CI, 73%-95%), 82% (74%-92%), 78% (67%-91%), and 46% (24%-87%), respectively (P=0.35). Median OS was NR for patients with 0, 1, and 2 HRCA and was 58.2 months (95% CI 38.8-NR) for patients with 3+ HRCA with 2-year OS of 93% (95% CI, 86%-100%), 96% (91%-100%), 89% (81%-99%), and 81% (60%-100%), respectively (P=0.13). In univariable analysis, lack of t(14;16) (HR 0.32, 95%CI 0.11-0.95; P=0.03), White race (HR 0.38, 95%CI 0.17-0.84; P=0.02), presence of ≤2 HRCA (HR 0.31, 95%CI 0.11-0.92; P=0.04) were associated with longer OS in this cohort of HR-NDMM patients treated with KRd induction. Multivariable analysis for clinical variables, including specific HRCA, number of HRCA present, age, gender, race, R-ISS stage, presence of EMD, presence of cPCs, cardiac history, and upfront ASCT did not demonstrate any single variable was a significant predictor of progression or death.
Conclusion: For this real-world patient population, KRd induction was associated with high response rates and promising outcomes with best ORR of 94% and mPFS of 6.5 years for patients with high-risk newly diagnosed multiple myeloma consistent with FORTE trial data. PFS did not significantly differ among HR patients with 0, 1, 2, 3+ HRCA although mPFS for patients with 3+ HRCA was only 17.5 months. Data with maintenance therapy and longer follow-up outcomes will be presented at the meeting.
Disclosures
Tan:Sanofi: Honoraria; Takeda: Research Funding; Janssen: Current Employment, Honoraria, Research Funding. Korde:CCO, OncLive, Intellisphere, Remedy Health: Consultancy; Janssen: Other: Advisory Board; Amgen, Janssen, Epizyme, AbbVie: Research Funding. Hultcrantz:Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding; Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria. Hassoun:Celgene, Takeda, and Janssen Pharmaceuticals: Research Funding. Mailankody:Allogene Therapeutics: Research Funding; Takeda Oncology: Research Funding; Physician Education Resource: Honoraria; Bristol Myers Squibb: Research Funding; MJH Life Sciences: Honoraria; Fate Therapeutics: Research Funding; Janssen Oncology: Research Funding; Janssen Oncology: Consultancy; Optum Oncology: Consultancy; OncLive: Honoraria; Legend Biotech: Consultancy; Caribou Therapeutics: Research Funding. Shah:C4 Therapeutics: Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board; Sabinsa: Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; M and M Labs: Research Funding; Plantable: Research Funding. Lesokhin:Janssen: Honoraria, Research Funding; Bristol Myers Squibb: Research Funding; ArcellX: Consultancy; Pfizer: Honoraria, Research Funding. Lahoud:MorphoSys Inc, Kite: Consultancy. Shah:BMS: Research Funding; Amgen: Research Funding; ArcellX: Other: DSMB; Beyond Spring: Research Funding; Janssen: Research Funding. Scordo:Medscape, LLC: Honoraria; Amgen, Inc.: Research Funding; Angiocrine Bioscience, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; CancertNetwork (Intellisphere LLC): Honoraria. Landau:Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding; Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Hofmeister:Sanofi: Research Funding; BMS: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees. Kaufman:Incyte: Consultancy; Sanofi: Consultancy; BMS: Consultancy; Abbvie: Consultancy. Nooka:Adaptive Biotechnologies, Amgen, BeyondSpring, Bristol Myers Squibb, Cellectar Biosciences, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, ONK therapeutics, Pfizer, Sanofi, Secura Bio, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Cellectis, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Kite Pharma, Merck, Pfizer, Takeda: Honoraria, Research Funding. Usmani:EdoPharma: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Moderna: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Array Biopharma: Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lonial:AbbVie Inc, Amgen Inc, Bristol-Myers Squibb Company, Celgene Corporation, Genentech, a member of the Roche Group, GlaxoSmithKline, Janssen Biotech Inc, Novartis, Pfizer Inc, Takeda Pharmaceuticals USA Inc: Consultancy, Other: Advisory Committee; Bristol-Myers Squibb Company, Janssen Biotech Inc, Novartis, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research, Research Funding; Novartis: Research Funding; Janssen: Research Funding; TG Therapeutics Inc: Other: Board of Directors with Stock. Joseph:BMS: Honoraria; Janssen Oncology: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal